Emicizumab represents a therapeutic innovation not only for haemophiliacs A with inhibitor but also for those without inhibitor. This is what the recently published Haven 3 study has shown. Thephase 3 study was performed on 152 enrolled patients aged ≥ 12 years and randomly assigned witha 2: 2: 1 ratio in 4 groups in order to evaluate prophylaxis with emicizumab. The first 3 groups included patients who had received episodic treatments (“on demand”) with factor VIII:
Group A. Patients were given subcutaneously (s.c.) emicizumab at the dose of 1.5 mg/kg of b.w. once a week.
Group B. Patients received emicizumab s.c. at the dose of 3 mg/kg of b.w. every 2 weeks.
Group C. Patients did not perform prophylaxis with emicizmab.
The primary end point was to evaluate the difference in the rate of bleeding/year (“Annualized Bleeding Rate”- ABR) of group A vs group B and group B vs group C.
Group D. To this group belonged patients who had received previously prophylaxis with factor VIII . They were given emicizumab s.c. at the maintenance dose of 1.5 mg / kg of b.w. once a week. Intra-individual comparisons were performed with patients who previously participated to the prospective non-interventional study (NIS) with the emicizumab.
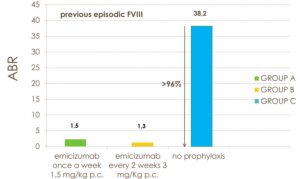
In summary, the data show that ABR had 1.5 events in group A (95% confidence interval [CI], 0.9 – 2.5); 1.3 events in group B (95% CI, 0.8 – 2.3); 38.2 events in group C (95% CI, 22.9 – 63.8), with a rate of 96% for group A and 97% for group B respectively (P <0.001 for both comparisons) (Fig.1).
A total of 56% of participants in group A and 60% of those in group B respectively had no treated bleeding when compared to patients in group C where all had treated bleeding.
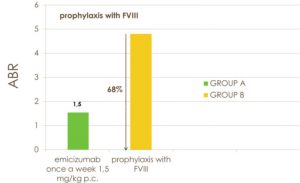
In the intra-individual comparison of 48 participants, prophylaxis with emicizumab resulted in a 68% lower ABR than in patients previously treated with factor VIII prophylaxis (P <0.001) (Fig. 2). No adverse events, excluding slight reactions at the injection site, neither episodes of thrombosis or thrombotic microangiopathy, nor development of anti-emicizumab antibodies or against factor VIII were observed.
In haemophiliacs A without inhibitor, subcutaneous administration of emicizumab once weekly or every 2 weeks has shown lower ABR than in hemophiliacs who did not use prophylaxis.
In addition, more than half of participants did not present treated bleeding. In the intra-individual comparison, treatment with emicizumab resulted in a significant reduction of ABR compared to prophylaxis with factor VIII, that is the standard treatment of hemophiliacs without inhibitor.
Suggested readings
J. Mahlangu, J. Oldenburg, I. Paz‐Priel, C. Negrier, M. Niggli, M.E. Mancuso, C. Schmitt, V. Jimenez‐Yuste, C. Kempton,C. Dhalluin, M.U. Callaghan, W. Bujan, M. Shima, J.I. Adamkewicz, E. Asikanius, G.G. Levy, and R. Kruse‐Jarres. N Engl J Med 2018;379:811-22.
Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med 2017; 377: 809-18.
Shima M, Hanabusa H, Taki M, et al. Factor VIII–mimetic function of humanized bispecific antibody in hemophilia A. N Engl J Med 2016; 374: 2044-53.



